

Fluorine is the most reactive non-metal in the Periodic Table. The halogens become less reactive down the Group. The compounds that halogens formed with metals are all ionic. Ion of fluorine is fluoride, ion of chlorine is chloride, ion of bromine is bromide and that of iodine is iodide. One electron is taken in to achieve stable noble gas octet electronic configuration. Each halogen atom has seven electrons in the valence shell.

Halogens form an ion with a charge of -1. A bright flame is observed in this reaction. For example, sodium burns in chlorine to form sodium chloride. Halogens are very reactive non-metals, they react with metals to form ionic salts. Chemical Properties Formation of ionic compound Fluorine is pale yellow, chlorine is pale yellow-green, bromine is reddish brown, while iodine is purplish black.Īll halogens are poisonous and must be handled carefully in the Chemistry lab. The colour intensity increases down the Group. Bromine exists as liquid, while iodine exists as solid.Īll the halogens are coloured. At room conditions, fluorine and chlorine exist as gases.
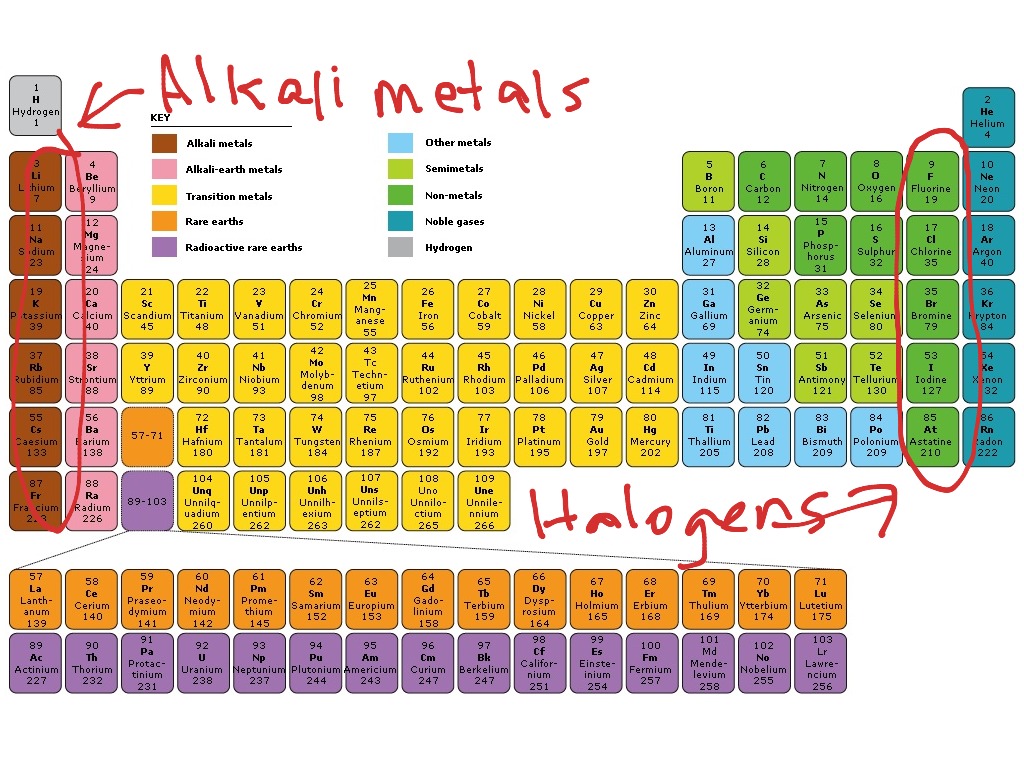
Their boiling points increase down the Group. Hence, the melting point and boiling point of halogens are low. The forces between the molecules are weak, little amount of energy is required to overcome this weak intermolecular forces of attraction. Each molecule is made of two atoms covalently bonded together, hence we call them diatomic molecules. Halogens are non-metals that exist as molecules. The elements in this Group are fluorine, chlorine, bromine, iodine and astatine. Group VII elements are called the halogens.


 0 kommentar(er)
0 kommentar(er)
